3D Microstructure of Soft Magnetic Elastomer Membrane
Soft magnetic elastomer membranes enable fast magnetic actuation under low fields. In our project, we… Read More
Events & Resources
News, Events and Resources from NXCT Partners
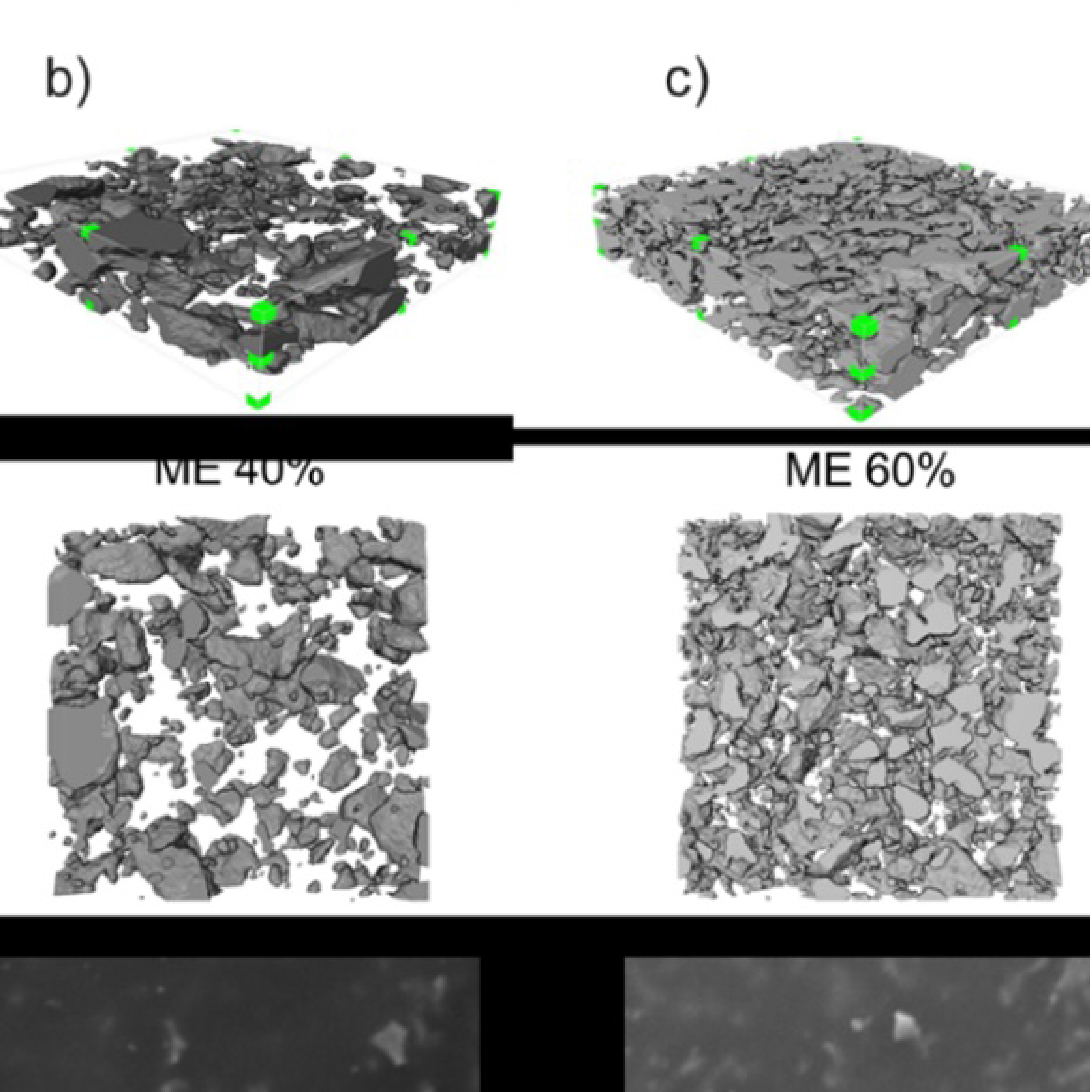
Three dimensional imaging of the development of a corrosion product that grows as a by product of the corrosion process on oil and gas pipelines. Observing when and where the internal features of the corrosion product develop i.e. porosity and pathways through the corrosion product.
Structural failure due to corrosion from the presence of CO2 presents a problem to the integrity of pipelines which must be carefully managed. There are inherent environmental and financial implications of using corrosion inhibitor to reduce corrosion rates of line pipe. However the corrosion may be partially mitigated by the formation and growth of a protective scale that is a by-product of the corrosion process. The degree of protectiveness a scale can offer depends on the morphology of the scale produced which in turn is dependant on the type of environment the steel is operating in.
Previous work in literature utilising FIB and SEM imaging has attempted to characterise the scale and suggests that once a complete and compact scale is formed the scale can be characterised as protective, but internal features such as porosity and internal pathways that link bulk solution to the metal surface may create ideal situations for promoting localised corrosion and cannot be observed with these traditional two dimensional imaging method.
The work carried out has involved corroding X-65 steel specimens in various corrosive solutions found within oil and gas pipelines; CO2 saturated, Anoxic, impurities i.e. NaCl, FeCl. The specimens were removed from the solution at time intervals of 1, 2, 5, 9, and 12 days and imaged using the Zeiss Xradia 810 Ultra in the Henry Moseley. The scale of the corrosion product formed is on the scale of 10um to 20um therefore the nano-resolution of the Ultra was the chosen XCT instrument.
The work completed here will help support knowledge of the corrosion product that forms and help determine whether the corrosion product can limit corrosion enough to remove the need to use inhibitors.
William Pearson, PhD Researcher, University of Manchester

Soft magnetic elastomer membranes enable fast magnetic actuation under low fields. In our project, we… Read More
Nowadays, the increasing capability of micro-manufacturing processes enables the manufacture of miniature products with extremely… Read More
Injection of CO2 into shale reservoirs to enhance gas recovery and simultaneously sequester greenhouse… Read More